atmospherics
|
Thanks, everybody. I realize that most of you have day jobs and don’t have time to be teachers in my self-designed Elder-Hostel Education program, but if you do have a moment, could you answer the following questions? In these questions, the words “cool” and “warm” will have a technical meaning: “cooler and warmer than layers of air at the same altitude.” This is to take account of adiabatic cooling and warming. Other things being equal, 100 degree air mass in ABQ will only be 85 in Santa Fe, but, in my sense of the term, the two have the same “warmness”. If somebody has a better term for two air masses that have an “adiabatically equivalent temperature”, I would love it. By the way, by “balloon” I think I have to mean a weightless sealed expandable enclosure. (1) Fill a balloon with oxygen; put it in a sealed vessel full of nitrogen, releasing enough nitrogen from the bottom of the vessel to compensate for the mass of the oxygen. Will the balloon sink or float? (2) Through the top of a vessel full of nitrogen, introduce gently a volume of oxygen, releasing enough nitrogen from the bottom to compensate for the mass of the introduced oxygen. Will the oxygen, remain on the surface, distribute itself through the nitrogen, or sink to the bottom? (3) Perform operation #1, introducing a balloon full of cool air into the top of a vessel containing air that is warm. Will the balloon sink or float? (4) Perform operation #2, gently introducing a volume of cool air into the top of a vessel full of warm air. Will the cool air, remain on the surface, distribute itself through the warm air, or sink to the bottom? (5) Perform operation #1, introducing a balloon full of dry air into the top of a vessel containing air that is moist (air with water vapor [mixed][dissolved] in it). Will the balloon sink or float? (6) Finally, perform operation #2, gently introducing a volume of dry air into the top of a vessel containing air that is moist (air with water vapor [mixed][dissolved] in it. Will the dry air, remain on the surface, distribute itself through the moist air, or sink to the bottom? I realize as I wrote these that the words change meaning in subtle ways as one moves from environment to environment. To say that a molecule is “cool” is to describe it’s behavior, not its identity. A similar confusion lurks in calling air “moist”. In case you are curious, this all to do with elevated mixing levels which are implicated in tornado formation. To put it bluntly, what holds an elevated mixing level up until it’s needed to cause a tornado? Nicholas S. Thompson Emeritus Professor of Psychology and Biology Clark University http://home.earthlink.net/~nickthompson/naturaldesigns/ ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
As, oddly, no one seems to have mentioned it yet... I'm pretty sure that
air does separate. Am I wrong to think that "air" at a high enough altitude is
mostly hydrogen? So the question is not what keeps it from separating, but what
keeps it from separating more fully... right?
Eric On Wed, Jun 13, 2012 01:13 AM, Steve Smith <[hidden email]> wrote: Nick -Eric Charles Professional Student and Assistant Professor of Psychology Penn State University Altoona, PA 16601 ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
Huh? That makes no sense. Moreover, there is NO hydrogen or helium in
our atmosphere. Any that we might have once had is long gone. Given the very large average height and correspondingly high average speed (and much higher speed in the high-speed tail of the distribution), these very-low-mass species can simply escape from Earth. More dramatically, the gravitational field of low-mass objects such as asteroids is too small to keep an atmosphere of any kind. Bruce On Wed, Jun 13, 2012 at 6:09 PM, ERIC P. CHARLES <[hidden email]> wrote: > As, oddly, no one seems to have mentioned it yet... I'm pretty sure that air > does separate. Am I wrong to think that "air" at a high enough altitude is > mostly hydrogen? So the question is not what keeps it from separating, but > what keeps it from separating more fully... right? > > Eric ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
In reply to this post by Eric Charles
You are correct, the air at the topmost level of the atmosphere, the
exosphere, is primarily composed of hydrogen. This hydrogen most likely comes from decomposition of atmospheric water into hydrogen and oxygen. You're also right that there is a gradient of gasses as you move from sea level to space. If you look at the basic principles, (excluding weather etc) you have diffusion mixing the gases while you have gravitational forces separating them. I'm not sure how much of the mixing we see is from diffusion and what is from other forces (such as weather). That'd be an interesting problem to look at. (Perhaps see how much experimentally measured gradients differs from what we calculate from a simple diffusion / gravity model. ) **************************** Greg Sonnenfeld On Wed, Jun 13, 2012 at 6:09 PM, ERIC P. CHARLES <[hidden email]> wrote: > As, oddly, no one seems to have mentioned it yet... I'm pretty sure that air > does separate. Am I wrong to think that "air" at a high enough altitude is > mostly hydrogen? So the question is not what keeps it from separating, but > what keeps it from separating more fully... right? > > Eric > > > > On Wed, Jun 13, 2012 01:13 AM, Steve Smith <[hidden email]> wrote: > > Nick - > > I'd like to interject here that your original question about the mixing (or > not) of atmospheric components was a very legitimate question... > > I hope (many) of the responses you got (Bruce's in particular) helped dispel > the mystery of what we all know circumstantially (though I'm not sure most > of us would notice if the O2 levels were elevated after a quiet, still > night?). > > While I may personally have some specific experience (as anecdotally > described) with the formalities of these problems I think it is assumed that > most of us here do not! > > The innocence of many of your questions as posed should be more overtly > valued... many of us are busy asking (quietly) similar or related > questions. > > Don't let the unregulated banter that follows some of your questions be > mistaken for anything but what it is, a good excuse for banter... Doug and I > perhaps being the worst of the crowd for that. > > So... I say let the discussion of mixtures and solutions and miscibility > continue... I have to admit that I have a "working" knowledge of miscibility > but not enough understanding of it's foundations! > > - Steve > > > SS wrote: > > > > But are you surprised that your bottle of wine, beer, or hard liquor hasn't > seperated before you even get to pour it? > > > > NST REPLIES: > > > > Well I guess I am surprised by that. Whiskey (etc) is just a mixture of > alcohol and water,no? I suspect that there is some sort of distinction > lurking here between a “solution” of something and a “mixture” of > something. > > > > > > > > From: [hidden email] [mailto:[hidden email]] On Behalf > Of Steve Smith > Sent: Tuesday, June 12, 2012 3:45 PM > To: The Friday Morning Applied Complexity Coffee Group > Subject: Re: [FRIAM] atmospherics > > > > Nick - > > I think Bruce just gave a good calibration on this with his great > description not only of why or why not to breathe Uranium Hexaflouride (cuz > you will have to stand on your head to empty it from your lungs!) but also > the relative density of the gasses in question. > > Try the analogy of mixed drinks. Every good bartender knows that you put > the alcohol into the glass first so that when you add the water-based stuff > (tonic, seltzer, juice, etc.) the two mix naturally. If you pour the > alcohol *over* the watery things, you risk the alcohol "floating" rather > than mixing. We could go into the implications of low and high "proof" > alcohol, etc. > > But are you surprised that your bottle of wine, beer, or hard liquor hasn't > seperated before you even get to pour it? > > AS I think Doug mentioned, thermal energy alone is a good mixer... even > without the constant stirring of wind and convection... > > - Steve > > Sorry. Mixed up the weight of N and O. So my question should have been, > Why don’t we wake up in a layer of oxygen on still nights? > > > > Which brings us to your question about what would make me expect that a > mixture would separate out into its lighter and heavier components. You > tell me! Other things being equal, don’t heavier things tend to sink when > mixed with lighter ones? > > > > N > > > > > > > > From: [hidden email] [mailto:[hidden email]] On Behalf > Of Douglas Roberts > Sent: Tuesday, June 12, 2012 2:43 PM > To: The Friday Morning Applied Complexity Coffee Group > Subject: Re: [FRIAM] atmospherics > > > > Let's not ignore temperature: my farts are a good 20 degrees F above > ambient (at present), and tend to rise before mixing into the unfortunate > nearby environs. And, just in case you were wondering what the composition > of a fart was: > > > > The major components of the flatus, which are odorless, by percentage > are:[4] > > § Nitrogen: 20–90% > > § Hydrogen: 0–50% > > § Carbon dioxide: 10–30% > > § Oxygen: 0–10% > > § Methane: 0–10% > > > > 4. ^ "Human Digestive System". Encyclopædia Britannica. Retrieved > 2007-08-22. > > > > --Doug > > > > On Tue, Jun 12, 2012 at 12:33 PM, Roger Critchlow <[hidden email]> wrote: > > Nick -- > > > > N2 weighs 28 gm/mole, O2 weighs 32 gm/mole, Ar weighs 40 gm/mole, CO2 weighs > 44 gm/mole, and H2O weighs 18 gm/mole. > > > > Why would anyone expect the lighter components of a mixture to fall down > more than the heavier ones? If anything, you'd expect the heavier ones to > concentrate toward the bottom. > > > > And why would anyone expect a mixture to spontaneously separate into pure > components? That happens in real life like where? > > > > As it happens, CO2 is the heaviest normal component and it does pool in > confined spaces often enough that CO2 alarms are available in hardware > stores. Propane, C3H8, weighs 44 gm/mole and is notorious for pooling in > confined spaces and then exploding, often in the bilge of a boat and > spectacularly. > > > > -- rec -- > > > > On Tue, Jun 12, 2012 at 10:44 AM, Nicholas Thompson > <[hidden email]> wrote: > > So, somebody asked me, in my role as a weather nerd, how come the nitrogen > in the atmosphere doesn’t all fall to the bottom on still nights and > suffocate us all. I asked the question of > stupid-answers-to-stupid-questions-asked-by-stupid-people.com and THEY said, > well, there’s just too much going on. N molecules and the O molecules are > just too busy, what with convection and windcurrents, and all, to separate, > even on still nights. Now, that business doesn’t prevent cold molecules of > Nitrogen and Oxygen to separate from warm ones, or wet ones (not sure what > that means) to separate from dry ones. I was hoping that somebody on FRIAM > could give some sort of a clue what kind of a mixture AIR is? It is > suddenly seeming kinda special. > > > > > > > > Nicholas S. Thompson > > Emeritus Professor of Psychology and Biology > > Clark University > > http://home.earthlink.net/~nickthompson/naturaldesigns/ > > http://www.cusf.org > > > > > > > > > > > ============================================================ > > FRIAM Applied Complexity Group listserv > > Meets Fridays 9a-11:30 at cafe at St. John's College > > lectures, archives, unsubscribe, maps at http://www.friam.org > > > > > > ============================================================ > FRIAM Applied Complexity Group listserv > Meets Fridays 9a-11:30 at cafe at St. John's College > lectures, archives, unsubscribe, maps at http://www.friam.org > > > ============================================================ > FRIAM Applied Complexity Group listserv > Meets Fridays 9a-11:30 at cafe at St. John's College > lectures, archives, unsubscribe, maps at http://www.friam.org > > Eric Charles > > Professional Student and > Assistant Professor of Psychology > Penn State University > Altoona, PA 16601 > > > > ============================================================ > FRIAM Applied Complexity Group listserv > Meets Fridays 9a-11:30 at cafe at St. John's College > lectures, archives, unsubscribe, maps at http://www.friam.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
You can start reading lots about our Atmosphere at:
http://en.wikipedia.org/wiki/Earth%27s_atmosphere At some point the article mentions that the troposphere (layer) is of uniform composition (but for water) and the exosphere is mainly hydrogen and helium. The latter is higher than the space station orbit while the former extends from sea level to up to 56,000ft (at the equator). I'm not sure what happens to the concentration gradients in between. Also of note is what happens to temperature as you go higher - there's a nice chart. See the extensive links to other articles. Thanks Robert C On 6/13/12 9:11 PM, Greg Sonnenfeld wrote: You are correct, the air at the topmost level of the atmosphere, the exosphere, is primarily composed of hydrogen. This hydrogen most likely comes from decomposition of atmospheric water into hydrogen and oxygen. You're also right that there is a gradient of gasses as you move from sea level to space. If you look at the basic principles, (excluding weather etc) you have diffusion mixing the gases while you have gravitational forces separating them. I'm not sure how much of the mixing we see is from diffusion and what is from other forces (such as weather). That'd be an interesting problem to look at. (Perhaps see how much experimentally measured gradients differs from what we calculate from a simple diffusion / gravity model. ) **************************** Greg Sonnenfeld On Wed, Jun 13, 2012 at 6:09 PM, ERIC P. CHARLES [hidden email] wrote:As, oddly, no one seems to have mentioned it yet... I'm pretty sure that air does separate. Am I wrong to think that "air" at a high enough altitude is mostly hydrogen? So the question is not what keeps it from separating, but what keeps it from separating more fully... right? Eric On Wed, Jun 13, 2012 01:13 AM, Steve Smith [hidden email] wrote: Nick - I'd like to interject here that your original question about the mixing (or not) of atmospheric components was a very legitimate question... I hope (many) of the responses you got (Bruce's in particular) helped dispel the mystery of what we all know circumstantially (though I'm not sure most of us would notice if the O2 levels were elevated after a quiet, still night?). While I may personally have some specific experience (as anecdotally described) with the formalities of these problems I think it is assumed that most of us here do not! The innocence of many of your questions as posed should be more overtly valued... many of us are busy asking (quietly) similar or related questions. Don't let the unregulated banter that follows some of your questions be mistaken for anything but what it is, a good excuse for banter... Doug and I perhaps being the worst of the crowd for that. So... I say let the discussion of mixtures and solutions and miscibility continue... I have to admit that I have a "working" knowledge of miscibility but not enough understanding of it's foundations! - Steve SS wrote: But are you surprised that your bottle of wine, beer, or hard liquor hasn't seperated before you even get to pour it? NST REPLIES: Well I guess I am surprised by that. Whiskey (etc) is just a mixture of alcohol and water,no? I suspect that there is some sort of distinction lurking here between a “solution” of something and a “mixture” of something. From: [hidden email] [[hidden email]] On Behalf Of Steve Smith Sent: Tuesday, June 12, 2012 3:45 PM To: The Friday Morning Applied Complexity Coffee Group Subject: Re: [FRIAM] atmospherics Nick - I think Bruce just gave a good calibration on this with his great description not only of why or why not to breathe Uranium Hexaflouride (cuz you will have to stand on your head to empty it from your lungs!) but also the relative density of the gasses in question. Try the analogy of mixed drinks. Every good bartender knows that you put the alcohol into the glass first so that when you add the water-based stuff (tonic, seltzer, juice, etc.) the two mix naturally. If you pour the alcohol *over* the watery things, you risk the alcohol "floating" rather than mixing. We could go into the implications of low and high "proof" alcohol, etc. But are you surprised that your bottle of wine, beer, or hard liquor hasn't seperated before you even get to pour it? AS I think Doug mentioned, thermal energy alone is a good mixer... even without the constant stirring of wind and convection... - Steve Sorry. Mixed up the weight of N and O. So my question should have been, Why don’t we wake up in a layer of oxygen on still nights? Which brings us to your question about what would make me expect that a mixture would separate out into its lighter and heavier components. You tell me! Other things being equal, don’t heavier things tend to sink when mixed with lighter ones? N From: [hidden email] [[hidden email]] On Behalf Of Douglas Roberts Sent: Tuesday, June 12, 2012 2:43 PM To: The Friday Morning Applied Complexity Coffee Group Subject: Re: [FRIAM] atmospherics Let's not ignore temperature: my farts are a good 20 degrees F above ambient (at present), and tend to rise before mixing into the unfortunate nearby environs. And, just in case you were wondering what the composition of a fart was: The major components of the flatus, which are odorless, by percentage are:[4] § Nitrogen: 20–90% § Hydrogen: 0–50% § Carbon dioxide: 10–30% § Oxygen: 0–10% § Methane: 0–10% 4. ^ "Human Digestive System". Encyclopædia Britannica. Retrieved 2007-08-22. --Doug On Tue, Jun 12, 2012 at 12:33 PM, Roger Critchlow [hidden email] wrote: Nick -- N2 weighs 28 gm/mole, O2 weighs 32 gm/mole, Ar weighs 40 gm/mole, CO2 weighs 44 gm/mole, and H2O weighs 18 gm/mole. Why would anyone expect the lighter components of a mixture to fall down more than the heavier ones? If anything, you'd expect the heavier ones to concentrate toward the bottom. And why would anyone expect a mixture to spontaneously separate into pure components? That happens in real life like where? As it happens, CO2 is the heaviest normal component and it does pool in confined spaces often enough that CO2 alarms are available in hardware stores. Propane, C3H8, weighs 44 gm/mole and is notorious for pooling in confined spaces and then exploding, often in the bilge of a boat and spectacularly. -- rec -- On Tue, Jun 12, 2012 at 10:44 AM, Nicholas Thompson [hidden email] wrote: So, somebody asked me, in my role as a weather nerd, how come the nitrogen in the atmosphere doesn’t all fall to the bottom on still nights and suffocate us all. I asked the question of stupid-answers-to-stupid-questions-asked-by-stupid-people.com and THEY said, well, there’s just too much going on. N molecules and the O molecules are just too busy, what with convection and windcurrents, and all, to separate, even on still nights. Now, that business doesn’t prevent cold molecules of Nitrogen and Oxygen to separate from warm ones, or wet ones (not sure what that means) to separate from dry ones. I was hoping that somebody on FRIAM could give some sort of a clue what kind of a mixture AIR is? It is suddenly seeming kinda special. Nicholas S. Thompson Emeritus Professor of Psychology and Biology Clark University http://home.earthlink.net/~nickthompson/naturaldesigns/ http://www.cusf.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org Eric Charles Professional Student and Assistant Professor of Psychology Penn State University Altoona, PA 16601 ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
And here's some (scary?) data on atmospheric CO2 trends which do not
showing a constant concentration with time.
http://www.esrl.noaa.gov/gmd/ccgg/trends/#mlo Annual fluctuations observed at Mauna Lao, Hawaii at 3500m are reported to be due to vegetation cycles suggesting something about atmospheric mixing. Thanks Robert C On 6/13/12 9:46 PM, Robert J. Cordingley wrote: You can start reading lots about our Atmosphere at: ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
In reply to this post by Greg Sonnenfeld
What means "atmosphere" anyway? For the purposes of this
discussion, I have been assuming "Troposphere" which is roughly
where most "interesting" phenomena happens... like human habitation,
most "life", most of what we call "weather", etc. The
"Stratosphere" is also interesting and important for lots of
reasons, but except for some jet (civilian and military) activity,
not much going on with humans or life (again, excepting bacteria and
some high flying birds, and the occasional Everest climber).
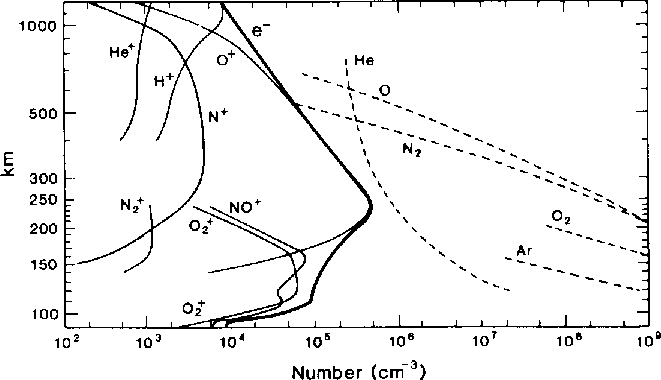
In the diagram below, the "troposphere" occupies the bottom .2mm or so... (10km).... Bruce sez "no H nor He" and Greg sez "Lots of H"... it just depends on how you define the "atmosphere". My colloquial preference is with Bruce on this... Troposphere and maybe Stratosphere.... the rest is "near space" to me.. polluted with various ions in densities high enough to bother orbiting (and re-entering) vehicles and affect the heating/cooling/UV load on the planet but otherwise irrelevant to the most of us. What was a gaseous mixture becomes more of a Plasma somewhere up there. 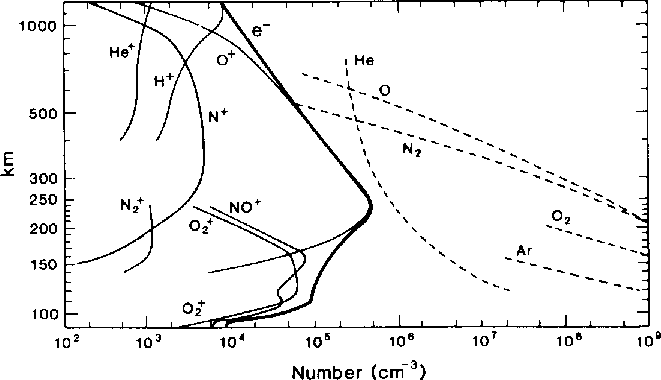 from:
http://www.physics.usyd.edu.au/~cairns/teaching/lecture16/node2.html
where there are formulae for those who need formulae along with pictures too. from my vague memory of my senior physics project.... most of the interesting questions for NASA were in the large range known on earth as the Thermosphere and Mesosphere... which to most of us here is definitely "upper atmosphere"... whaYou are correct, the air at the topmost level of the atmosphere, the exosphere, is primarily composed of hydrogen. This hydrogen most likely comes from decomposition of atmospheric water into hydrogen and oxygen. You're also right that there is a gradient of gasses as you move from sea level to space. If you look at the basic principles, (excluding weather etc) you have diffusion mixing the gases while you have gravitational forces separating them. I'm not sure how much of the mixing we see is from diffusion and what is from other forces (such as weather). That'd be an interesting problem to look at. (Perhaps see how much experimentally measured gradients differs from what we calculate from a simple diffusion / gravity model. ) **************************** Greg Sonnenfeld On Wed, Jun 13, 2012 at 6:09 PM, ERIC P. CHARLES [hidden email] wrote:As, oddly, no one seems to have mentioned it yet... I'm pretty sure that air does separate. Am I wrong to think that "air" at a high enough altitude is mostly hydrogen? So the question is not what keeps it from separating, but what keeps it from separating more fully... right? Eric On Wed, Jun 13, 2012 01:13 AM, Steve Smith [hidden email] wrote: Nick - I'd like to interject here that your original question about the mixing (or not) of atmospheric components was a very legitimate question... I hope (many) of the responses you got (Bruce's in particular) helped dispel the mystery of what we all know circumstantially (though I'm not sure most of us would notice if the O2 levels were elevated after a quiet, still night?). While I may personally have some specific experience (as anecdotally described) with the formalities of these problems I think it is assumed that most of us here do not! The innocence of many of your questions as posed should be more overtly valued... many of us are busy asking (quietly) similar or related questions. Don't let the unregulated banter that follows some of your questions be mistaken for anything but what it is, a good excuse for banter... Doug and I perhaps being the worst of the crowd for that. So... I say let the discussion of mixtures and solutions and miscibility continue... I have to admit that I have a "working" knowledge of miscibility but not enough understanding of it's foundations! - Steve SS wrote: But are you surprised that your bottle of wine, beer, or hard liquor hasn't seperated before you even get to pour it? NST REPLIES: Well I guess I am surprised by that. Whiskey (etc) is just a mixture of alcohol and water,no? I suspect that there is some sort of distinction lurking here between a “solution” of something and a “mixture” of something. From: [hidden email] [[hidden email]] On Behalf Of Steve Smith Sent: Tuesday, June 12, 2012 3:45 PM To: The Friday Morning Applied Complexity Coffee Group Subject: Re: [FRIAM] atmospherics Nick - I think Bruce just gave a good calibration on this with his great description not only of why or why not to breathe Uranium Hexaflouride (cuz you will have to stand on your head to empty it from your lungs!) but also the relative density of the gasses in question. Try the analogy of mixed drinks. Every good bartender knows that you put the alcohol into the glass first so that when you add the water-based stuff (tonic, seltzer, juice, etc.) the two mix naturally. If you pour the alcohol *over* the watery things, you risk the alcohol "floating" rather than mixing. We could go into the implications of low and high "proof" alcohol, etc. But are you surprised that your bottle of wine, beer, or hard liquor hasn't seperated before you even get to pour it? AS I think Doug mentioned, thermal energy alone is a good mixer... even without the constant stirring of wind and convection... - Steve Sorry. Mixed up the weight of N and O. So my question should have been, Why don’t we wake up in a layer of oxygen on still nights? Which brings us to your question about what would make me expect that a mixture would separate out into its lighter and heavier components. You tell me! Other things being equal, don’t heavier things tend to sink when mixed with lighter ones? N From: [hidden email] [[hidden email]] On Behalf Of Douglas Roberts Sent: Tuesday, June 12, 2012 2:43 PM To: The Friday Morning Applied Complexity Coffee Group Subject: Re: [FRIAM] atmospherics Let's not ignore temperature: my farts are a good 20 degrees F above ambient (at present), and tend to rise before mixing into the unfortunate nearby environs. And, just in case you were wondering what the composition of a fart was: The major components of the flatus, which are odorless, by percentage are:[4] § Nitrogen: 20–90% § Hydrogen: 0–50% § Carbon dioxide: 10–30% § Oxygen: 0–10% § Methane: 0–10% 4. ^ "Human Digestive System". Encyclopædia Britannica. Retrieved 2007-08-22. --Doug On Tue, Jun 12, 2012 at 12:33 PM, Roger Critchlow [hidden email] wrote: Nick -- N2 weighs 28 gm/mole, O2 weighs 32 gm/mole, Ar weighs 40 gm/mole, CO2 weighs 44 gm/mole, and H2O weighs 18 gm/mole. Why would anyone expect the lighter components of a mixture to fall down more than the heavier ones? If anything, you'd expect the heavier ones to concentrate toward the bottom. And why would anyone expect a mixture to spontaneously separate into pure components? That happens in real life like where? As it happens, CO2 is the heaviest normal component and it does pool in confined spaces often enough that CO2 alarms are available in hardware stores. Propane, C3H8, weighs 44 gm/mole and is notorious for pooling in confined spaces and then exploding, often in the bilge of a boat and spectacularly. -- rec -- On Tue, Jun 12, 2012 at 10:44 AM, Nicholas Thompson [hidden email] wrote: So, somebody asked me, in my role as a weather nerd, how come the nitrogen in the atmosphere doesn’t all fall to the bottom on still nights and suffocate us all. I asked the question of stupid-answers-to-stupid-questions-asked-by-stupid-people.com and THEY said, well, there’s just too much going on. N molecules and the O molecules are just too busy, what with convection and windcurrents, and all, to separate, even on still nights. Now, that business doesn’t prevent cold molecules of Nitrogen and Oxygen to separate from warm ones, or wet ones (not sure what that means) to separate from dry ones. I was hoping that somebody on FRIAM could give some sort of a clue what kind of a mixture AIR is? It is suddenly seeming kinda special. Nicholas S. Thompson Emeritus Professor of Psychology and Biology Clark University http://home.earthlink.net/~nickthompson/naturaldesigns/ http://www.cusf.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org Eric Charles Professional Student and Assistant Professor of Psychology Penn State University Altoona, PA 16601 ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
Okay, there are issues of definitions. I'll note that the upper atmosphere is bombarded by "cosmic rays" which in fact are mostly very high-energy protons originating outside our Solar System. Protons are of course the nuclei of hydrogen....
Bruce ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
In reply to this post by Steve Smith
Ah i was mentioning the exosphere because it was very relevant to the question about layering/mixing of gases. Hydrogen is on the most outer layer as would be expected. I do imagine alot of this comes from space ions as well.
**************************** Greg Sonnenfeld “Two h's walk into a bar. The first one says, "What is this? Some kind of physics joke?” On Wed, Jun 13, 2012 at 10:51 PM, Steve Smith <[hidden email]> wrote:
============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
|
Another example of a top-of-the-atmosphere special layer is of course the ozone (O3) layer, continually produced by ultraviolet light but unstable. These "surface" phenomena don't have anything to do with how nitrogen, oxygen, and uranium hexafluoride are distributed throughout the atmosphere.
Bruce
On Thu, Jun 14, 2012 at 1:35 AM, Greg Sonnenfeld <[hidden email]> wrote: Ah i was mentioning the exosphere because it was very relevant to the question about layering/mixing of gases. Hydrogen is on the most outer layer as would be expected. I do imagine alot of this comes from space ions as well. ============================================================ FRIAM Applied Complexity Group listserv Meets Fridays 9a-11:30 at cafe at St. John's College lectures, archives, unsubscribe, maps at http://www.friam.org |
| Free forum by Nabble | Edit this page |